This article was originally published by the American Urological Association and has been edited for the web. Authors include Kristin Brogaard, PhD, Co-founder and CSO of Inherent Biosciences; TIm Jenkins, PhD, Brigham Young University; and Larry Lipschultz, MD, Baylor College of Medicine.
Male infertility is a growing concern in the United States. It affects an estimated 7 million men and plays a causative role in nearly 50% of infertility cases.1 However, despite the size of the global fertility market, estimated at $26 billion, there remain few diagnostics tests related to male fertility. The lack of testing and innovation in the male fertility space carries several negative consequences, including the underdiagnosis of infertility in men, leading to female partners undergoing unnecessary invasive procedures and costing these couples both money and time.2
Additionally, poor male reproductive health, evidenced by impaired semen parameters, is highly associated with poor overall health in men.3,4 Poor overall health in men includes increased metabolic diseases, cancers, cardiovascular disease, and shorter lifespans.3,4 Indeed, there is a fundamental lack of research and funding to better understand this association of morbidities and the role of semen as a substrate for men’s health screening.5
The Use of mTESE in Cases of Non-Obstructive Azoospermia
A particularly under-supported and under-researched subset of infertile men are those with non-obstructive azoospermia (NOA). NOA indicates there are no visible sperm in the semen with no identifiable obstruction of the urogenital tract. NOA affects approximately 1% of men worldwide or about 39.7 million men.
In the cases where men present with NOA, a procedure called microscopic testicular sperm extraction (mTESE) can be utilized. mTESE isolates sperm directly from the testes, giving these men a chance to use their own sperm to conceive. mTESE is a highly invasive surgery that opens a man’s testicles and microscopically searches for pockets of sperm. Despite being an extraordinary advancement in reproductive technology, mTESE procedures have very high costs for patients. On average, mTESE procedures cost $12,000 out of pocket, are highly invasive, and physicians fail to find any sperm in 40-50% of procedures.4
Currently, there are limited tools to assess if a patient will have sperm in their testes, and if they are a good candidate for the mTESE procedure. Consequently, the lack of technology to predict mTESE success has led to unsuccessful procedures, poor outcomes, and significant medical bills. New technology is needed to better assess the treatment paradigm for using mTESE.
Research Supports a Less Invasive Assessment
In the data presented at the American Urological Association (AUA), we described the initial research of a new, noninvasive, semen-based technique to predict presence or absence of sperm in the NOA testicle. Overall, the goal of this research is to lead to a novel semen assessment to predict mTESE success.
This new technique identifies sperm-derived cell-free DNA (cfDNA) by identifying specific DNA methylation patterns in testicular tissue or semen that are only found on sperm. The methylation patterns are interrogated using an Oxford Nanopore MinION DNA sequencer.
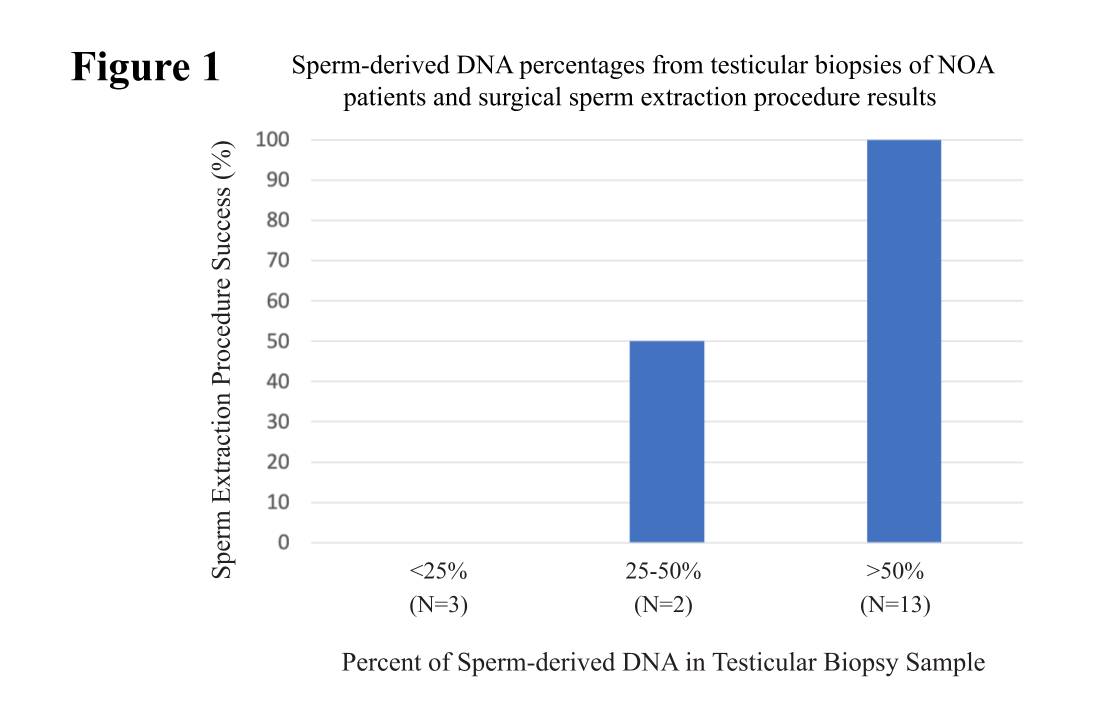
Initially, we analyzed 18 testicular biopsy samples with varying underlying histopathology. We found no sperm found in the testicular tissue of men with <0.25% sperm-derived DNA. Comparatively, 100% of men with >50% sperm-derived DNA in testicular tissue had sperm in their testicles (Figure 1).
To assess if we could have the same predictability from seminal plasma, we analyzed 14 blinded semen samples from men with varying testicular sperm extraction results. In this cohort of patients with <5% sperm-derived DNA in their seminal plasma, there was a 0% success rate of sperm extraction (N=4).
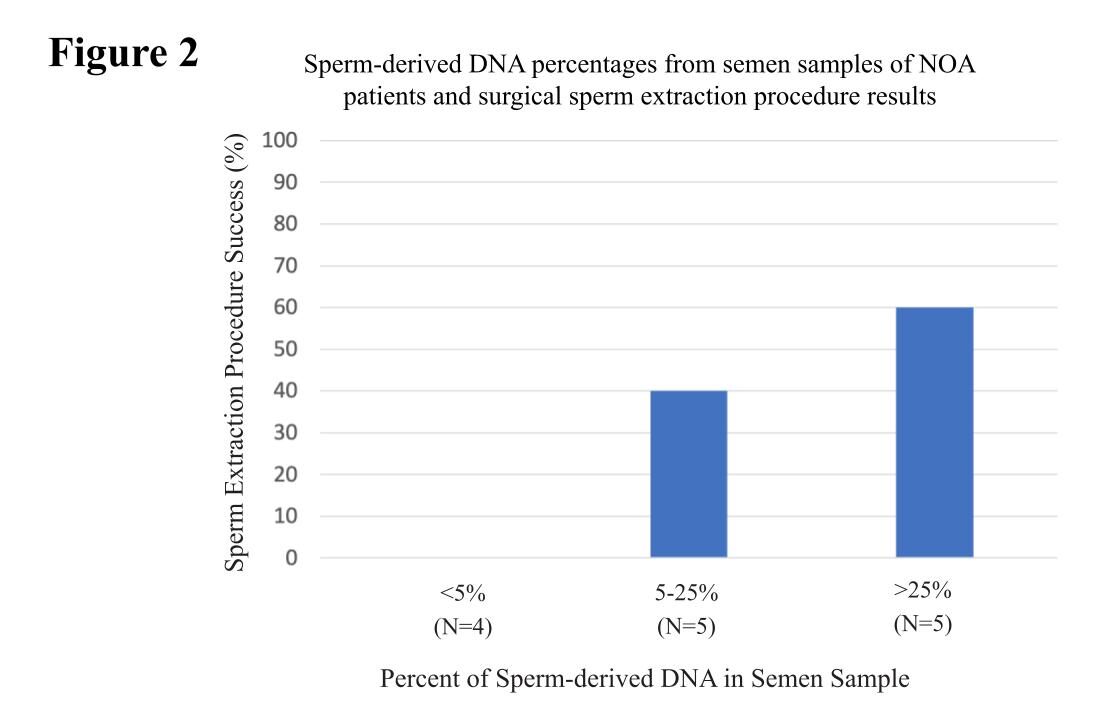
Furthermore, patients with >25% sperm-derived DNA in the seminal plasma had a 60% success rate with sperm extraction (N=5) (Figure 2). Evidently, these data suggest men with <5% of sperm-derived DNA in their seminal plasma should not undergo a sperm extraction procedure.
The Future of NOA and mTESE
More samples are needed for continued analysis. However, these data show semen as a promising analyte for predicting success of sperm extraction and ultimately saving patients both unnecessary physical and economic hardship.
References
1) Fertility Services Market Size, Trends & Growth Opportunities (2022-2028). Zion Market Research. 23 June 2022 [cited 6 Sep 2022]. Available: https://www.zionmarketresearch.com/report/fertility-services-market
2) Turner KA, Rambhatla A, Schon S, Agarwal A, Krawetz SA, Dupree JM, et al. Male infertility is a women’s health issue-research and clinical evaluation of male infertility is needed. Cells. 2020;9: E990. doi:10.3390/cells9040990. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7226946/
3) Ahrenfeldt LJ, Möller S, Wensink MJ, Eisenberg ML, Christensen K, Jensen TK, et al. Impaired fecundity as a marker of health and survival: a Danish twin cohort study. Hum Reprod Oxf Engl. 2021;36: 2309-2320. doi:10.1093/humrep/deab077. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8496092/
4) Tharakan T, Luo R, Jayasena CN, Minhas S. Non-obstructive azoospermia: current and future perspectives. Fac Rev. 2021;10: 7. doi:10.12703/r/10-7. Available: https://pubmed.ncbi.nlm.nih.gov/33659925/
5) De Jonge C, Barratt CLR. The present crisis in male reproductive health: an urgent need for a political, social, and research roadmap. Andrology. 2019;7: 762-768. doi:10.1111/andr.12673. Available: https://pubmed.ncbi.nlm.nih.gov/31241256/